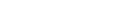The South African Health Products Authority (Sahpra) vigilance manager Mafora Matlala stressed on Wednesday that monitoring the safety of Covid-19 vaccines and communicating any risks is a critical priority for the regulatory body, assuring South Africans that the vaccines are safe to use.
The Government Communication and Information System, the National Press Club and the Sahpra hosted a webinar hoping to educate the public on the safety of Covid-19 vaccines.
The regulatory authority continuously monitors and evaluates the safety, efficacy and performance profile and management of any risk throughout the life cycle of all health products in the country.
“With the new Covid-19 vaccines that we currently have in the country, we all know that these are new products, therefore, we continuously need to monitor the risk benefit profile. So by so saying what we are doing is that we ensuring that risks are lesser and benefits are actually more of this product,” said Matlala.
South Africa authorised the use of four Covid-19 vaccines – Pfizer, the Sinovac, the AstraZeneca and Johnson & Johnson – but only Pfizer and Johnson & Johnson are being rolled out.
Matlala explained that there is careful and ongoing review of data submitted by pharmaceutical companies based on trials that have been performed.
Sahpra continuously reviewes international literature, safety databases and regulatory decisions made elsewhere.
The regulatory also encourages reporting of suspected adverse reactions and adverse events following immunisation in the country.
THE IMPORTANCE OF TAKING THE COVID-19 VACCINE
Matlala explained that the reason the public is encouraged to take the Covid-19 vaccine is that when looking at the adverse events received so far in the system, there are very few – about 2 000 – compared to the doses administered which sits at about 9.5-million as of Tuesday.
“What is important to highlight is that the serious AEFIs [Assessment, Evaluation, Feedback, & Intervention System] are extremely rare for the Covid-19 vaccines based on currently available evidence. Most of the AEFIs received are non-serious and mild, e.g. headache, pain and redness at the injection site, fever and chills,” she explained.
She went on to say that the benefits of the vaccines outweigh the risk.
National Immunisation Safety Expert Committee chairperson professor Hannelie Meyer said people should not assume that all reactions are due to the Covid-19 vaccines.
“We are currently vaccinating millions of people and there are many other diseases happening at the same time. You could be incubating another infectious disease at the same time and that you are being vaccinated,” added Meyer.
Sahpra will continue to monitor and inform health professionals and the public of up to date safety profiles of Covid-19 vaccines.
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here