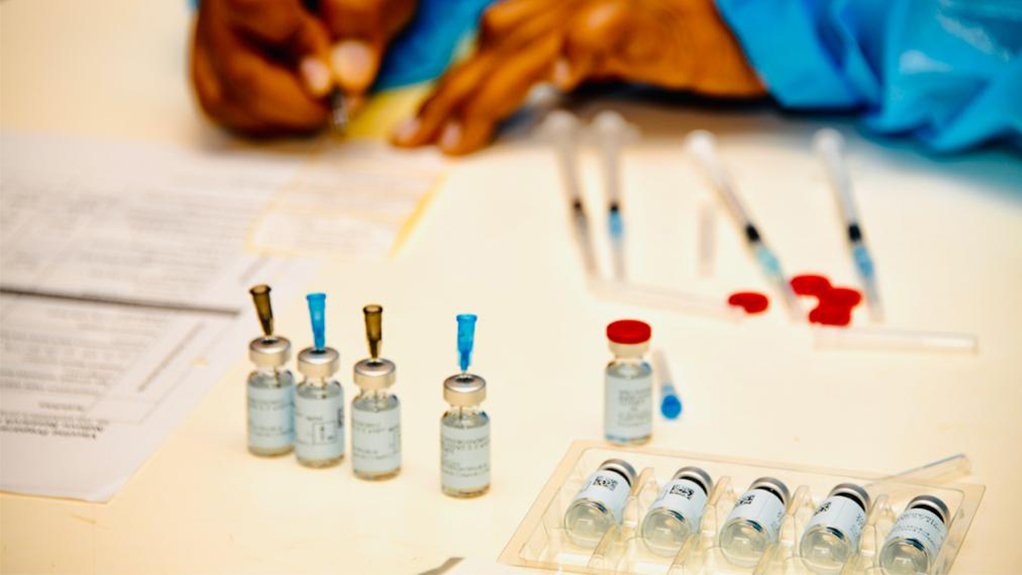
The South African Health Products Regulatory Authority (Sahpra) has announced the authorisation of Pfizer’s Comirnaty vaccine and the Covid-19 vaccine MC PHARMA, also known as Sinopharm/BIBP vaccine for use in South Africa.
Sahpra says the approval is based on acceptable safety, quality and efficacy data submitted by companies.
Sahpra CEO Dr Boitumelo Semete-Makokotlela pointed out that both vaccines have been registered in terms of Section 15 of the Medicines and Related Substance Act (Act 101 of 1965 as Amended), with conditions.
The Comirnaty vaccine was registered on January 25 with acceptable safety, quality and efficacy data submitted by Pfizer Laboratories to Sahpra as a rolling submission over the period February 3, 2021 to January 17, 2022.
The Comirnaty is an mRNA vaccine, indicated for active immunisation to prevent Covid-19 in individuals 12 years of age and older.
Sahpra explained that it is recommended that the second dose of this vaccine be administered three weeks after the initial dose.
Meanwhile, the Covid-19 vaccine MC Pharma was registered on January 31 following a rolling submission from MC Pharma to Sahpra.
The Covid-19 vaccine MC Pharma is an inactivated Vero Cell vaccine, indicated for immunisation against SARS-CoV-2 in those aged 18 years and older.
The vaccine is a two doses injection, administered at an interval of 2 to 4 weeks.
“Sahpra will continue to play its part in ensuring the quality, safety and efficacy of all health products, including all vaccines, to ensure that the South African republic is protected at all times,” Semete-Makokotlela said.
Other Covid-19 vaccines currently under various stages of review by Sahpra include vaccines from Sputnik V, Sinopharm and Novavax.
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here