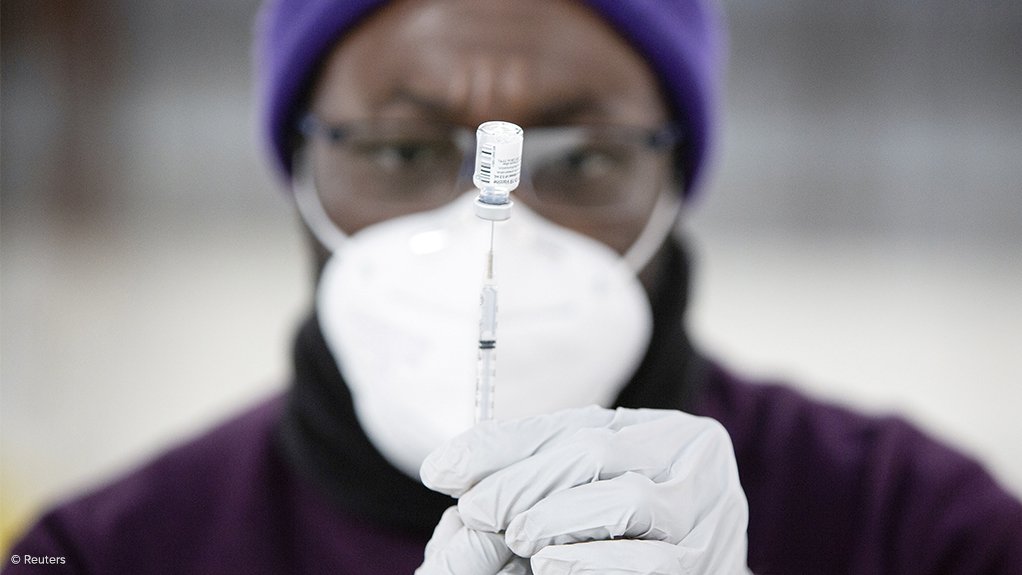
Cabinet has welcomed the recommendation by the South African Health Products Regulatory Authority (SAHPRA) regarding the lifting of the temporary pause placed on the Johnson & Johnson (J&J) Sisonke Vaccination Programme, but did not immediately announce an end to the suspension following its Wednesday meeting, which was also the first physical meeting of Cabinet since the introduction of the Covid-19 lockdown in 2020.
Acting Minister in The Presidency, Khumbudzo Ntshavheni, confirmed on Thursday that Cabinet had left it to Health Minister Dr Zweli Mkhize to announce the actual recommencement details, which would be made “as soon as the health department is ready with all the systems".
The recommencement was likely to be imminent, however, as Cabinet had been informed that a new consignment of one-million J&J vaccine doses would be delivered to government by Monday, April 26.
These doses were being manufactured at the Aspen facility, located in the Eastern Cape.
The Sisonke trial of the J&J vaccine was initiated in February to vaccinate health workers, after the AstraZeneca vaccine, initially procured for the purpose, was found to be ineffective against mild cases of the variant of Covid-19 first found in South Africa and which had emerged as the dominant variant in the country by early 2021.
However, the South African government placed a temporary halt on the administering of the J&J vaccine on April 14, after the US Food and Drug Administration and the Centers for Disease Control and Prevention paused the vaccine’s use in America so as to further investigate the relationship between the vaccine and the development of extremely rare blood clots.
The reviewed data confirmed that South Africa had not experienced any rare blood clots, with more than 292 600 health workers having been vaccinated under the Sisonke Vaccination Programme ahead of the pause.
On April 19, SAHPRA recommended that the pause in the Sisonke study be lifted, provided that specific conditions were met, including strengthened screening and monitoring of participants who are at high risk of a blood clotting disorder.
In addition, SAHPRA said measures should be implemented to ensure the safe management of any participants who develop vaccine-induced thrombosis and thrombocytopenia, while information sheets and informed consent forms would be updated to reflect the possible risk of a blood clotting disorder after vaccination.
The various updated documents, procedures and study arrangements were submitted to SAHPRA for approval, but resumption of the Sisonke study also required approval from the relevant Research Ethics Committees.
Cabinet said that the temporary suspension in South Africa was in line with government’s commitment to ensuring that comprehensive safety measures were undertaken regarding the vaccine roll-out.
Cabinet also welcomed the successful negotiation of an additional 10-million doses of the two-dose Pfizer vaccine, bringing the total doses of the Pfizer vaccine procured to 30-million.
It also welcomed the progress in the signing of the contract with J&J on the procurement of vaccines for the roll-out of Phase 2 of the vaccination programme, which would initially focus on South Africans above the age of 60, before being extended to essential workers and people living in congregate settings.
The establishment of the No-Fault Compensation Fund, in line with contractual agreements entered into with the pharmaceutical companies supplying South Africa with Covid-19 vaccines, was also approved.
“The Fund is also important for the protection of South Africans who may be affected by side effects of vaccines to access better support and treatment,” Cabinet said in a statement, reporting that the fund would be established through amendments to Section 27 of the Disaster Management Act, and would be chaired by a retired judge.
EMAIL THIS ARTICLE SAVE THIS ARTICLE ARTICLE ENQUIRY
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here